
Here, we focus on a different type of modification strategy, in which the polypeptide backbone is altered. These different modification strategies can be implemented in tandem. (4) Decoration: nonpeptide moieties (e.g., a carbohydrate, a fluorophore, a synthetic polymer) can be attached to side chains or termini. (3) Augmentation: polypeptide segments can be grafted onto the prototype protein to confer or enhance characteristics that are necessary for the intended application. (2) Truncation: portions of the polypeptide that are not necessary for the properties of interest can be removed. (1) Mutation: the side chain can be altered at one position, or multiple side chains can be altered. Starting from a prototype protein, researchers have traditionally had access to only a few types of modification. Such mimics can be used as research tools, diagnostic agents, or medicines some applications require the introduction of properties that are not manifested by the original protein. Scientists often seek molecules that mimic only a subset among the properties of a particular protein. Proteins evolve to display a specific set of properties that are advantageous to the organism in which they are produced.
Successes include mimicry of BH3 domains found in proapoptotic proteins, which leads to ligands for anti-apoptotic Bcl-2 family proteins, and mimicry of the gp41 CHR domain, which leads to inhibition of HIV infection in cell-based assays. Thus, these unnatural oligomers can be a source of antagonists of undesirable protein–protein interactions that are mediated by natural α-helices, or agonists of receptors for which the natural polypeptide ligands are α-helical. These α/β-peptides can mimic the informational properties of α-helices involved in protein–protein recognition events, as documented in numerous crystal structures. If the β content reaches 25–30% of the residue total, and the β residues are evenly distributed along the backbone, then substantial resistance to proteolytic degradation is often observed. These oligomers contain both α- and β-amino acid residues (α/β-peptides). Bending is introduced by a strong (i, i + 8) hydrophobic interaction between the side chains of N-terminal tryptophan and leucine at the middle of the peptide sequence.We describe a general strategy for creating peptidic oligomers that have unnatural backbones but nevertheless adopt a conformation very similar to the α-helix. The peptide alpha-helix is bent despite the lack of an amphipathic sequence. The relative topologies of the charged side chains demonstrate flexibility and overall compromised favorable medium/long-range electrostatic interactions. The NMR structural ensemble demonstrates competition between E-R/K (i, i + 4) and (i, i - 1) ion pair formation, with the (i, i - 1) interactions being dominant. The presence of E-R/K (i, i + 4) ion pairs was expected to enhance the stability of the alpha-helix by introducing favorable electrostatic interactions at the side chain level, in addition to the characteristic backbone (i, i + 4) hydrogen bonds. The peptide sequence was designed using amino acids that have propensity for alpha-helix specificity. In addition, our data are consistent with the presence of several other transient and interconverting conformers.
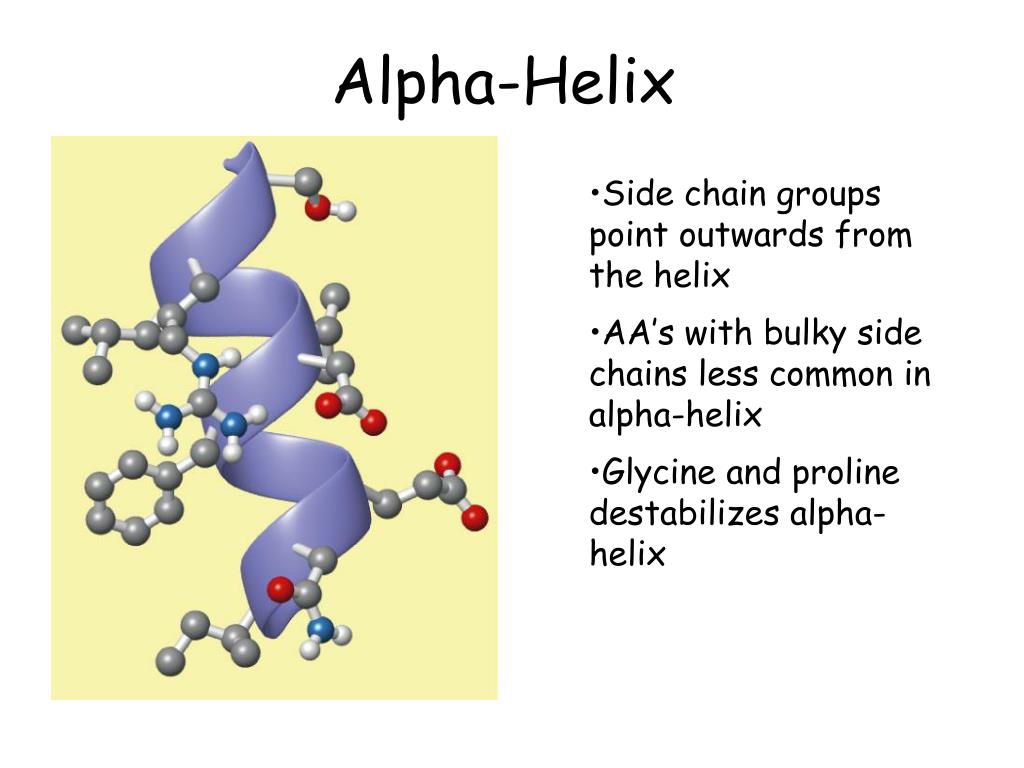
The peptide has a high population of a stable alpha-helical structure in the middle with fraying ends. We present the solution structure determination of a peptide with sequence Ac-WEAQAREALAKEAQARA-NH2, using NMR data.


 0 kommentar(er)
0 kommentar(er)
